Environment & Energy
Related: About this forumSupercritical CO2 Isolation of Lanthanides (Rare Earths) from Canadian Ores.
The paper I'll discuss in this post is this one: Technoeconomic Analysis of the Supercritical Fluid Extraction Process for the Extraction of Rare Earth Elements from Ores Gisele Azimi, Maziar E. Sauber, and Sicheng Li Industrial & Engineering Chemistry Research 2025 64 (16), 7993-8011
(Note: I hate it that the lanthanide elements are commonly called "rare earths" since many of them aren't all that rare, just as I hate the common reference to alkenes as olefins, but like many things in my life, this is a losing battle.)
Famously, it response to the blank stupidity of the orange slime mold infecting the White House, China, which is the world supplier of lanthanide elements, critical to most modern technology, has threatened to ban export to the United States, along with supplies of other critical elements they control, like gallium. While China controls most of the world's reserves, ex-China reserves are known, in the case of the paper under discussion, in Canada, another country that hates the orange slime mold infecting the White House as people on this web site do.
It is notable that used nuclear fuels can be considered an ore of the light lanthanides up to and including gadolinium, particularly after separation of the light actinides and some fission products in a fluoride volatility method. (A discussion of isolation of lanthanides as fluorides is available in the next issue of this journal, but I haven't gotten to it.)
The chemistry of isolation of lanthanides from oxide ores (they are present as oxides in most used nuclear fuels) is rather dirty to the extent that it relies on solvent extraction using organic solvents like kerosene. This paper refers to the use of supercritical carbon dioxide for this purpose. A supercritical fluid, for those not familiar with the terminology, is one that above a certain pressure, unsurprisingly termed the "critical pressure," Pc, and the "critical temperature," Tc, there is no distinction between a gas and liquid phases, as shown in a phase diagram:

As the introductory text from the paper explains, for carbon dioxide, this state is easily accessible since the temperatures and pressures are comparatively mild. The introduction:
Supercritical fluid extraction (SCFE) has emerged as an environmentally friendly technology for extracting metals, particularly REEs. (5) Developed in the late 1970s, SCFE has achieved commercial success across a range of industries, including energy applications like direct coal liquefaction (6) and enhanced oil recovery, (7) as well as food and pharmaceutical sectors, such as coffee decaffeination. (8) This widespread adoption highlights SCFE’s high scalability and advanced Technology Readiness Levels (TRLs). Supercritical carbon dioxide (sc-CO2) is a popular solvent in SCFE due to its inertness, cost-effectiveness, and favorable critical properties (Tc = 31.1 °C, Pc = 7.37 MPa, density = 469 kg/m3), (9) which allow for efficient recycling or venting with minimal waste. Its gas-like low viscosity and high solvability make sc-CO2 a versatile solvent for extracting metals from various materials. In certain cases, phase modifiers like methanol are added to enhance its solvability. However, sc-CO2’s nonpolar nature necessitates the use of complexing agents, such as tributyl phosphate (TBP), to facilitate effective extraction by neutralizing charges and improving metal ion interactions. TBP, a widely used organophosphorus compound, has demonstrated success in extracting REEs from synthetic oxides, nitrates, (10) and natural resources like zircon, (5) monazite, (11) and bastnäsite, (12) as well as secondary sources such as nickel–metal hydride batteries, (13) neodymium–iron-boron magnets, (14,15) fluorescent lamp phosphors, (3) lithium-ion batteries, (16) and polymeric waxes. (17)
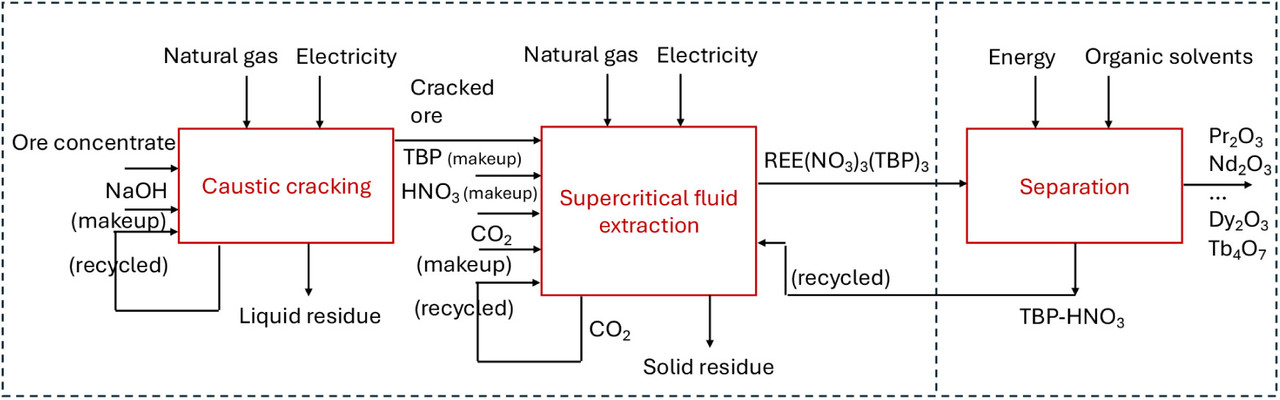
A recent study employed a complex Canadian REE ore as the primary material, comprising minerals such as zircon, allanite, monazite, bastnäsite, and synchisite. (5) A SCFE technique was developed, utilizing sc-CO2 as the solvent and a tributyl phosphate-nitric acid (TBP-HNO3) adduct as the chelating agent. To optimize the extraction process and achieve nearly 100% efficiency in REE recovery, the ore underwent a preparatory NaOH cracking treatment. Since this study proves that SCFE is promising for the extraction REEs from complex ores, a technoeconomic analysis is essential to assess the economic feasibility of the process. It can also provide valuable insights for guiding research efforts by pinpointing critical factors influencing profitability and identifying opportunities for optimization to facilitate the process’s industrial implementation.
In a previous study, our group performed a technoeconomic analysis of the SCFE process for the extraction of REEs from neodymium iron boron magnets and fluorescent lamps. (18) The results showed that the SCFE of REEs from secondary resources is profitable when the concentration of valuable REEs such as neodymium, dysprosium, and terbium is high in these resources. Besides this study, economic analyses related to SCFE are limited, and existing studies primarily focus on its application in organic material extraction...
The supercritical CO2 can be recovered simply by lowering the pressure. It is very clean chemistry that avoids organic solvents, many of which are petroleum derived.
Some figures from the text:
The caption:
The caption:
The caption:
The caption:
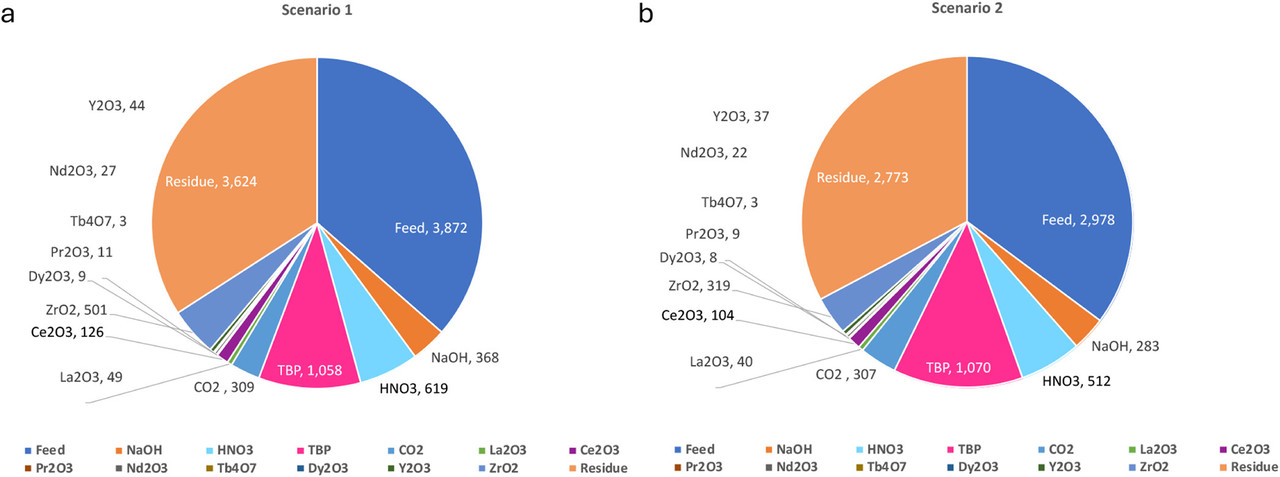
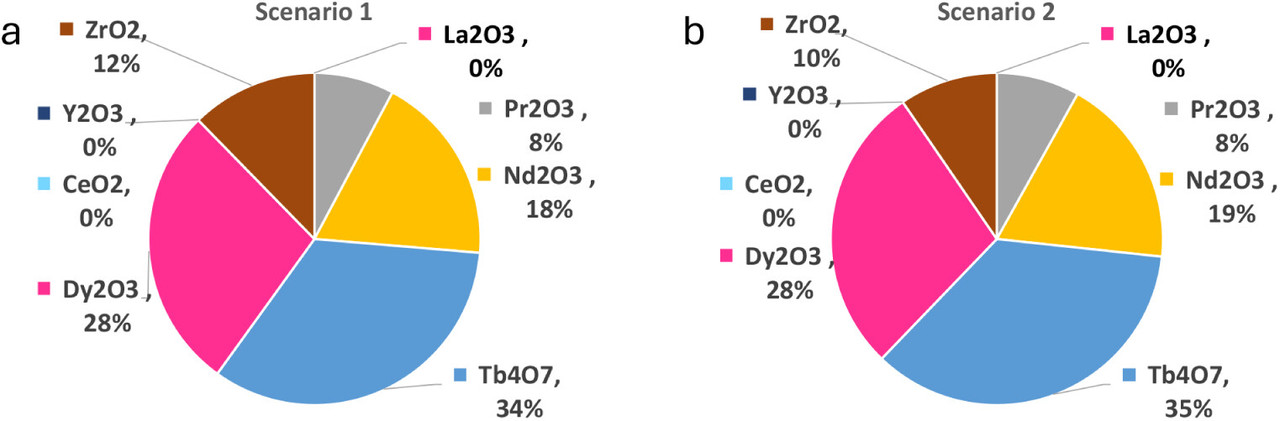
One is notably struck that these Canadian Ores contain the heavy lanthanides, notably dysprosium, an important additive for stabilizing the permanent magnetism of iron-neodymium boride magnets. Many lanthanide ores are relatively depleted with respect to the heavy lanthanides, the transgadolinium lanthanides, of which dysprosium is probably the most important.
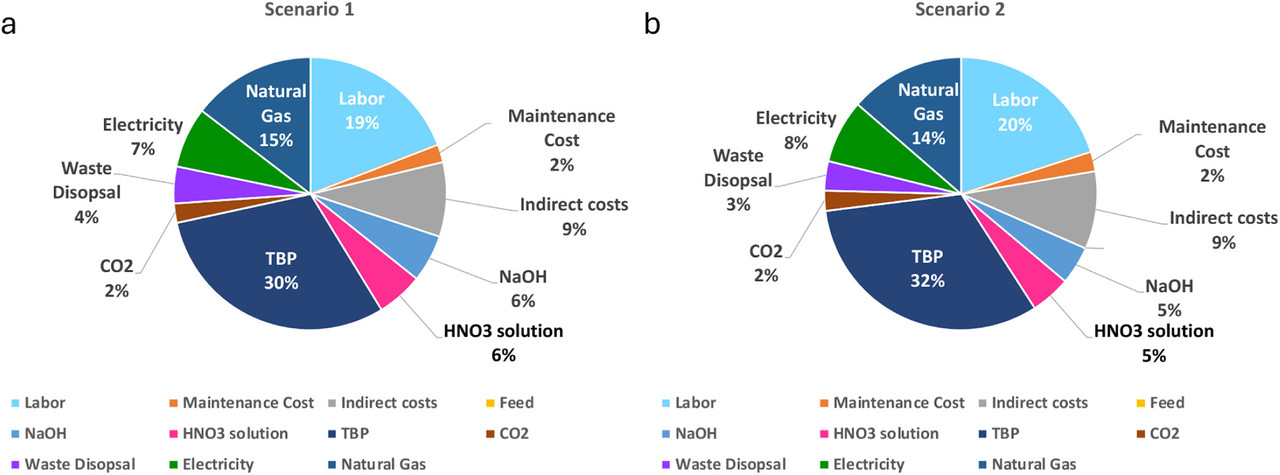
The paper contains significant details on the economics of a supercritical carbon dioxide lanthanide processing plant using two detailed scenarios; I will not have time to discuss them at any length here.
Let me just jump to the paper's conclusion:
This study demonstrates the economic viability of SCFE under specific conditions and provides a roadmap for scaling and optimizing the process. Strategic improvements in material usage, particularly TBP reduction, and continued refinement of cost estimates will be crucial to advancing this technology toward industrial adoption. Future efforts should also focus on pilot-scale validation, integration with existing refining operations, and comprehensive life cycle assessments to quantify environmental impacts.
I trust you are enjoying the weekend.
WhoaDude
(8 posts)Sure you have the right forum? ![]()
NNadir
(35,718 posts)...forum.
However again, magnetism is a very important tool for environmental management, and the chemistry associated with extraction of ores for their constituents is a very, very, very exigent environmental issue.
Thanks for your comment.